A guide to EC1935/2004 food contact materials regulations
Each person consumes approximately 1.9 kgs of food per day. For the population as a whole, that’s a staggering 5.2 trillion kilos annually. But, on its way from farm to fork, food comes into contact with a myriad of materials, each of which could present a potential source of contamination. Reducing the risk of contamination is critical to ensuring food safety. This starts with a full and proper understanding of the current legislation, and how to comply.
Contents
EC1935/2004 legislation: Meaningless or meaningful?
EC1935/2004 food contact materials (FCM) regulation was introduced in Europe in 2004 to ensure food safety. It applies to 17 groups of materials that come into contact with food during its production, processing, storage, preparation or serving. For example, equipment used in manufacturing, containers for food transportation and packaging materials.
However, there is complacency among many suppliers and manufacturers. A lack of standardisation in enforcement across European countries coupled with seemingly complex requirements means many disregard the legislation or make unintentional mistakes. Furthermore, on the work floor, downtime, efficiency and costs are the most important factors.
Yet, food safety is also vital and should be a priority as safety incidents have cost companies many millions of Euros. For example, in 2016 Mars recalled 20 million chocolate bars across 55 counties at an eye-watering cost of €12 million after a consumer found a piece of plastic in just one of its products! The plastic was traced back to a factory in the south of The Netherlands, where it was found to have come from a protective cover used in the manufacturing process.
A closer look at EC1935/2004
Prior to 2004, requirements for FCMs in Europe varied significantly from country to country, with each government having its own local rules and regulations. The European Commission recognised that a new single piece of legislation was required to standardise the regulation of FCMs and in 2004, EC1935/2004 regulation was established. The regulation is commonly referred to as the ‘Framework Regulation’. It was implemented in 2012 and it has been in effect since 2016.
There are two main requirements for FCM and articles as laid down in Article 3 of Regulation (EC) No 1935/2004.
First, all FCM and articles should be manufactured in compliance with good manufacturing practice, so that, under normal and foreseeable conditions of use they do not:
- transfer their constituents to food in quantities that could endanger human health
- bring about an unacceptable change in the composition of the food (e.g. taste or odour)
- bring about a deterioration of its organoleptic (acting on, or involving the use of, the sense organs) characteristics.
Second, the labelling, advertising and presentation of a FCM or article should not mislead the consumers.
Good manufacturing practice (GMP) is further defined in Regulation (EC) No 2023/2006, which applies to all materials and articles intended to come into contact with food. Under the GMP regulation, business operators are obliged to establish, implement and adhere to ‘quality assurance’ and ‘quality control’ systems.
It is worth noting that the term FCM includes direct or indirect contact and does not cover fixed public or private water supply equipment.
What does EC1935/2004 mandate?
The EC1935/2004 regulation defines requirements with regards to materials, quality (which is achieved through good manufacturing practice EC2023/2006), traceability, migrations tests and documentation.
EC1935/2004 provides legislation on the general application for 17 groups of materials, such as adhesives, metals, ceramics, cork, glass, plastics, paper, rubber and wood. On contact with food, each of these materials has the potential to alter the food and effect its quality. Depending on their composition and properties, materials may behave differently at various temperatures or on contact with certain foods and transfer their constituents to the food in a process known as ‘migration of substances’. For example, acidic foods can corrode metals and the resulting chemical elements might transfer into the food, posing a risk if ingested.
To avoid the risk of contamination, EC1935/2004 stipulates that the transfer of substances from the material to the food must remain within particular tolerances and there are set migration limits.
Full traceability of the materials is essential to facilitate control, to recall defective products, to provide consumers with information and to attribute responsibility. You need systems and procedures in place to identify businesses that supply and receive materials, and this information needs to be made available to the competent authorities on demand. Furthermore, the materials and articles need to be identifiable by an appropriate system which allows their traceability by means of labelling or relevant documentation.
Proper certification is key
Each material needs to have appropriate certification to comply with the regulation and the certificate needs to include specific information. Every certificate should include data as shown in figure 1, including material and traceability data, Declaration of Compliance (DOC) statement and Food and Drugs Administration (FDA) statement. Migration test results and stickers must also be present on the certificate for it to be valid. What’s more, certificates must be obtained before any component is placed on the market.
The regulations apply to each specific component material and part that comes into direct contact with food. For example, every O-ring in a machine will need a certificate for the different material compounds. And each time an O-ring is replaced by one made from a different material batch, you need a new certificate according to its batch. Don’t underestimate the amount of components that require a certificate, even the bearing lubricants have to be food safe.
Additionally, the necessity for certification depends on the final location of the machine, rather than the origin of the equipment. And, the responsibility for adhering to legislation not only lies with those manufacturing food, but also those manufacturing equipment for use in food processing
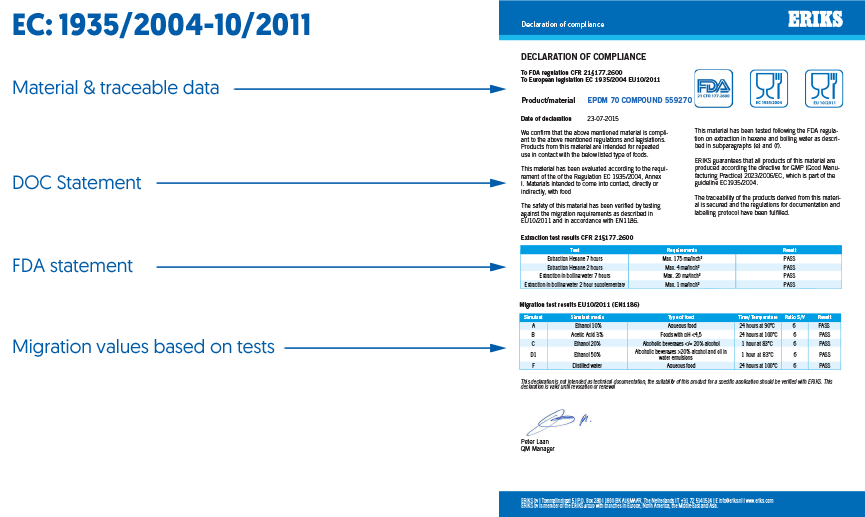
Figure 1 – Information that must be included on a certificate to comply with EC1935/2004
What are EC1935 & related frameworks?
Since EC1935/2004 is a framework regulation, it references other legislation that also applies and should be observed as shown in figure 2. For example, GMP EC2023/2006 as mentioned earlier must also be adhered to.
Four categories of FCMs are subject to additional specific regulations: plastic monomers and additives, active/intelligent materials (‘AIMs’), recycled plastic processes and regenerated cellulose film (RCF).
Plastic monomers and additives are governed by Regulation EU10/2011 which also became legally effective in May 2012 and sets out the safety requirements for plastics and articles intended to come into contact with food. This regulation stipulates that the plastic materials must not cause changes to the food, which must be demonstrated by means of a migration test which is outlined in EEC 82/711, EEC 93/8 and EEC 9748. Different tests apply to different foodstuffs ranging from aqueous food to alcoholic food and fatty food. An example of the migration values based on tests is shown in figure 3.
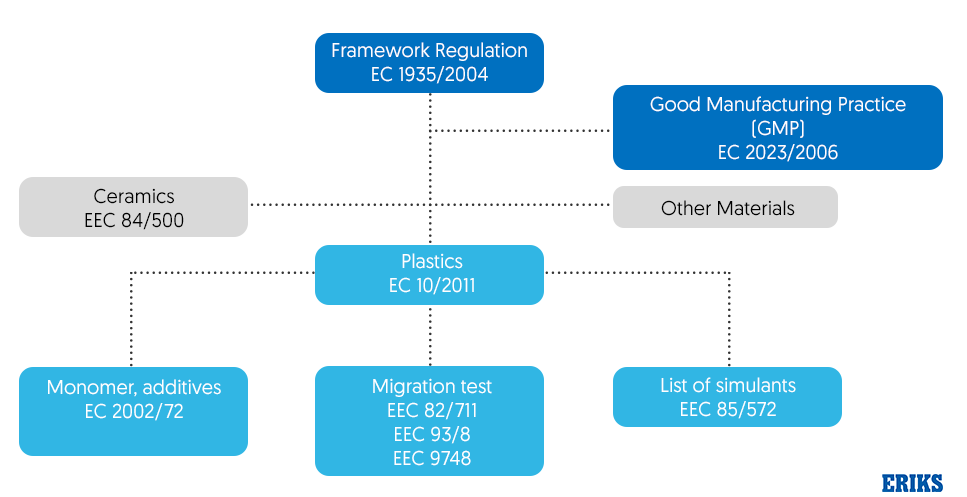
Figure 2 – How Framework Regulation EC 1935/2004 relates to other legislation
The manufacturer provides the conditions of operation, for example, type of food, temperature, contact time, number of contacts and contact surface, and tests must be carried out as per EN 1186 standard at an ISO17025 laboratory.
One material group that is not controlled by EU 10/2011 is rubber and silicon. There is no EU harmonization for these materials and instead they must comply with applicable law in the EU Member State in which they are operating. Alternatively, they are also in compliance if it meets the requirements as set out by article 3 of the EC1935/2004.
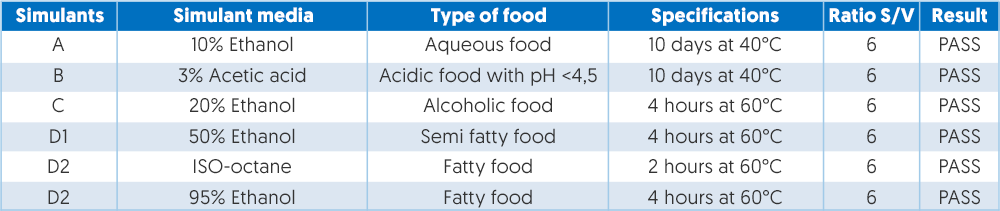
Figure 3 – Example of migration values based on test.
This article states that:
- Food contact materials should be manufactured according to the GMP, so that they do not transfer their constituents in quantities that could:
- Endanger human health, or;
- Bring about an unacceptable change in the composition of the food, or;
- Bring deterioration in the organoleptic characteristics of the food.
- Labelling, advertising, and presentation of material or article cannot mislead consumers.
Other international standards to be aware of when working with food and supplying equipment to the industry are those set by the U.S. FDA and the NHFPC China Food Safety Law, who govern food safety standards in other major markets.
It is worth bearing in mind, that even though you may be conforming to FDA standards, it does not mean you are necessarily adhering to EC1935/2004. Indeed, you need to pay special attention to this European regulation.
EC1935/2004 in practice
Complying with EC1935/2004 may appear daunting initially, but with some dedicated consideration can be achieved readily. There are a few steps that you can take to ease the process.
- Create a food safety team
Creating a food safety team (across quality, purchasing, warehousing, production) will pay dividends for ensuring appropriate measures are reviewed and put in place. Ensuring that food safety becomes part of a company’s culture means everyone throughout the business is aware of its importance and has safety front-of-mind.
- Simulate a recall
It is worth simulating recall events as this is a stark reminder of just how much time, effort and cost is needed to rectify a safety incident. It is a valuable exercise in reminding all of the disruption a recall would cause as well as providing practice in case an event should occur
- Check existing certificates
Be aware of the existing certificates and check whether they comply with the regulations. For example, do they contain all the pertinent information and are the migration values correct?
- Store certificates and DOCs
Finally, storing and managing certificates and DOCs appropriately is essential in case there is an audit. Being able to access them quickly when needed provides peace of mind, knowing that you fully abide by the regulations.
Working with trusted partners, who have extensive knowledge and experience with the technical and regulatory aspects of EC1935/2004 can help. Specialists can provide you with simple advice on material assessments, to more detailed guidance on how to achieve compliance, so you navigate the legislation with ease.
Contact
Direct contact
Want to speak directly with one of our employees or specialist? Don’t wait and get in touch
General contact
Get in contact with us. Please fill in our online form and we will contact you as soon as possible.

